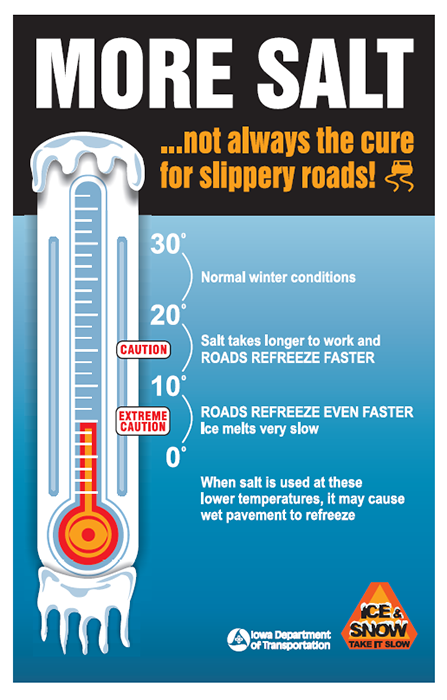
Cold Temperatures Reduce Salt's Effectiveness for Roadway De-icing
De-icing chemicals work by lowering the freezing point of water. Before a dry de-icing chemical can act, it must dissolve into a brine solution. The necessary moisture can come from snow on the road surface or from water vapor in the air (humidity).
Changing ice or snow into water requires heat from the air, sun, pavement or traffic friction. Even when the pavement is below freezing it holds some heat and can help melt snow and ice.
As air and pavement temperatures drop, the amount of salt needed to melt a given quantity of ice increases. Topography also plays a factor in pavement temperature and freezing and melting conditions. Shaded areas (by banks, vegetation, overhead bridges, and structures) are the first to freeze and last to melt because of their lower pavement temperatures.
It is also important to note that applying chemicals during blowing snow and cold temperatures will cause drifting snow to stick to the pavement, thus creating hazardous travel conditions.
Some things are truly dependent on Mother Nature. Sunshine and warmer temperatures are essential to getting the roadways back to normal winter driving conditions after an event involving significant snow or ice/snow and chilly temperatures.
Until the temperatures are such that salt is effective, snow plow trucks continue work to prepare the roadways by plowing the layer of snow and applying sand where necessary. When temperatures rise and salt application resumes, travelers can expect slushy conditions on the roadways and patches of packed snow/ice. Drivers should adjust their speed for conditions and not use cruise control.